
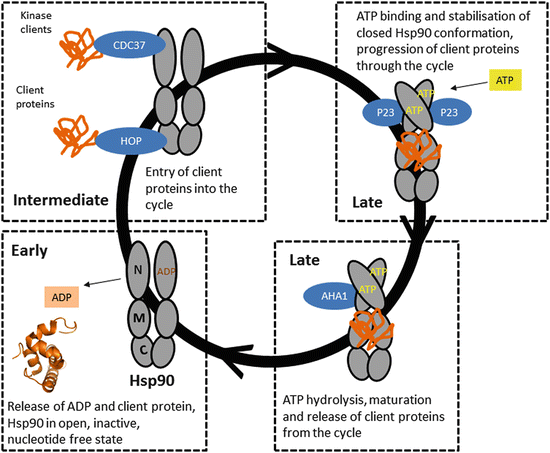
Hsp90 has been implicated in mediating the folding of a specific set of substrate proteins, Hsp90 is an abundant and essential molecular chaperone present in all eukaryotic cells. This chaperone system to maintain protein homeostasis in the cell. Hsp90 chaperone network should allow the determination of the mechanisms employed by The systematic application of large scale approaches to map out the Yeast Saccharomyces cerevisiae, Hsp90 was shown to interact directly or indirectly with at leastġ0% of the yeast ORFs. That Hsp90 plays a central role effecting multiple pathways and cellular processes. Interaction networks emerging from these large scale efforts clearly demonstrate Network of this chaperone in yeast and mammalian systems using the latest available proteomicĪnd genomic tools. Order to understand that complexity, several groups have attempted to map out the interaction Hence, Hsp90 function is highly complex in That regulate the activity of this chaperone. The functional cycle of the Hsp90 system requires a cohort of cochaperones and cofactors In eukaryotes,Ĭytoplasmic Hsp90 is absolutely essential for cell viability under all growth conditions. Hsp90 is an essential and ubiquitous molecular chaperone that is required for the properįolding of a set of client proteins at a late stage in their folding process.


 0 kommentar(er)
0 kommentar(er)
